
Genova is a global biopharmaceutical company that aims to innovate, develop and produce novel therapeutic protein drugs for the treatment of viral infections, cancer and autoimmune diseases. With its proprietary platform technologies, Genova’s operations include laboratory-based innovation of novel molecules, development of pilot production processes and commercial drug production, development and marketing. Genova has established a state of the art research center in North America, an engineering center for the development of pilot production processes in Beijing, a GMP-certified commercial production facility in Tsingtao and clinical research and business operation centers in Japan, Hong Kong and Vancouver.
Research and Development
Genova established a basic research center in North America in 2001. Genova’s proprietary platform technologies for engineering high-potency protein molecules and long-acting protein molecules have been utilized in the invention of novel molecules (Novaferon, Nova-EPO and Nova-GM-CSF) that have been patented and are currently in various stages of drug development.
In 2001, Genova also established an engineering center for the development of pilot production processes in Beijing. The engineering center has the capacity to develop production processes for recombinant proteins involving bacteria, yeast, and mammalian cell-lines.
In 2008, Genova established comprehensive pre-clinical and clinical research programs in North America and Asia.
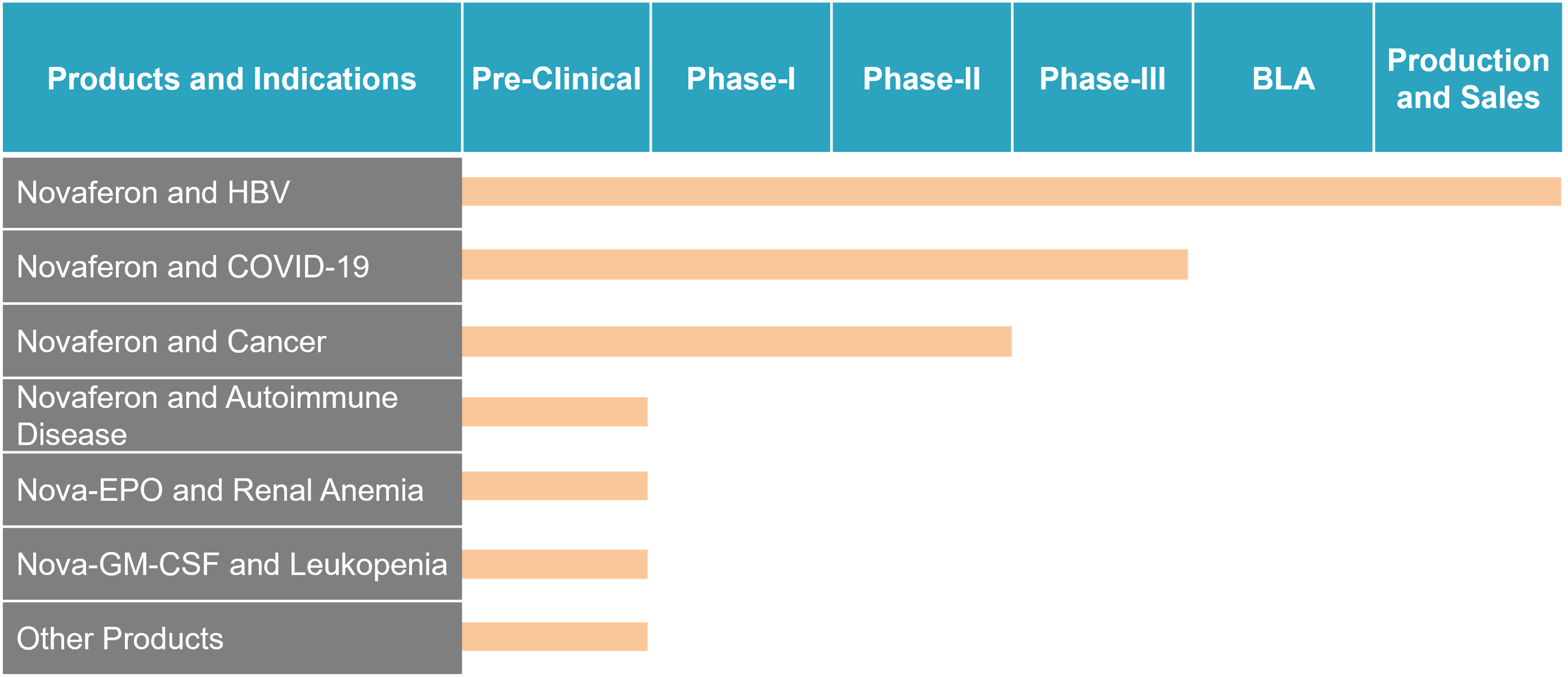
In 2018, NDA was granted for the application of Novaferon to hepatitis B treatment in China.
Genova’s ongoing research and development programs include clinical studies on Novaferon as an antiviral agent for COVID-19 treatment, and pre-clinical studies on Nova-EPO and Nova-GM-CSF.

Technic Platform
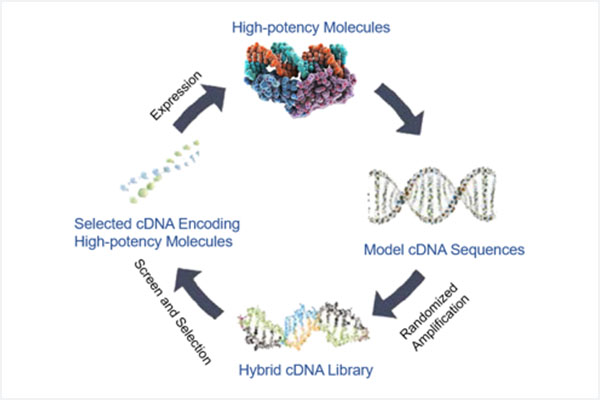
This illustration summarizes Genova’s proprietary technical platform for engineering high-potency protein molecules. This technology allows Genova to efficiently create and select novel protein molecules, and has been utilized in the invention of novel molecules that specifically exhibit enhanced activities against viral infections and tumor cells and immunomodulating functions. Novaferon, which is a novel protein molecule that exhibits these features, was invented using this technical platform.
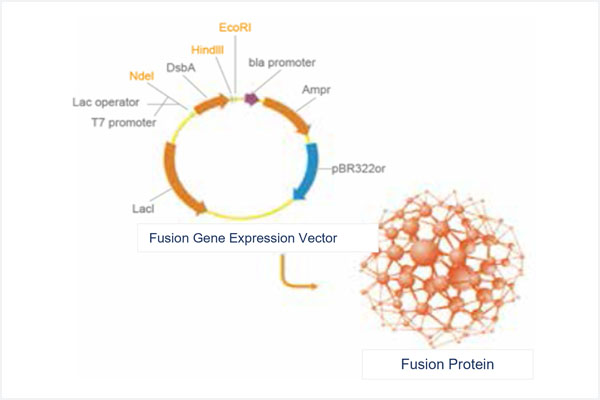
This illustration summarizes Genova’s proprietary technical platform for engineering protein molecules with prolonged blood half-life. This technology engages a novel mutated Fc fragment of human IgG, and allows for the fusion of a target polypeptide with this patented Fc fragment to form novel fusion protein molecules with prolonged blood half-life. It can be applied to create novel fusion molecules containing any selected target polypeptide. For all injectable protein drugs, the extension of half-life in circulation allows for reduced injection intervals and better patient compliance. This is eagerly welcomed in clinical practice. After Genova’s first long-acting protein drug has completed its clinical development and been approved globally, this proprietary technical platform is expected to be recognized as a general technology for developing other long-acting protein drugs.
Products
Novaferon
Novaferon exhibits potent antiviral activities against viruses and tumor cells and has enhanced immunomodulating functions. This novel protein molecule was created with Genova’s proprietary high-potency protein engineering technology.
Nova-EPO/Nova-GM-CSF
Nova-EPO and Nova-GM-CSF are novel protein molecules, created through the fusion of either EPO or GM-CSF polypeptide, respectively, with mutated Fc fragment of human IgG. These novel protein molecules exhibit significantly prolonged blood half-life. Nova-EPO and Nova-GM-CSF were created with Genova’s proprietary technology for engineering long-acting proteins.
Development Pipeline
Manufacturing facility
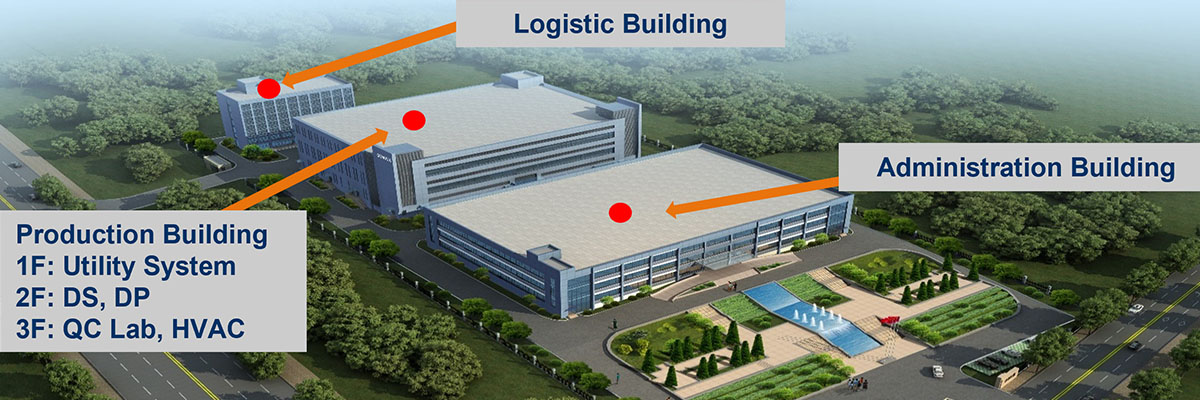
Genova’s first GMP-certified manufacturing facility is a state of the art facility, with nearly 30,000 square meters of floor area, and an annual production capacity of up to 100 million doses of Novaferon.
In June 2021, German experts conducted an EMA Qualified Person Inspection of the manufacturing facility and issued a formal QP certificate to verify that the facility meets the GMP standards of the European Union.
